Flowtron DVT
Uniform compression covering a wide variety of needs
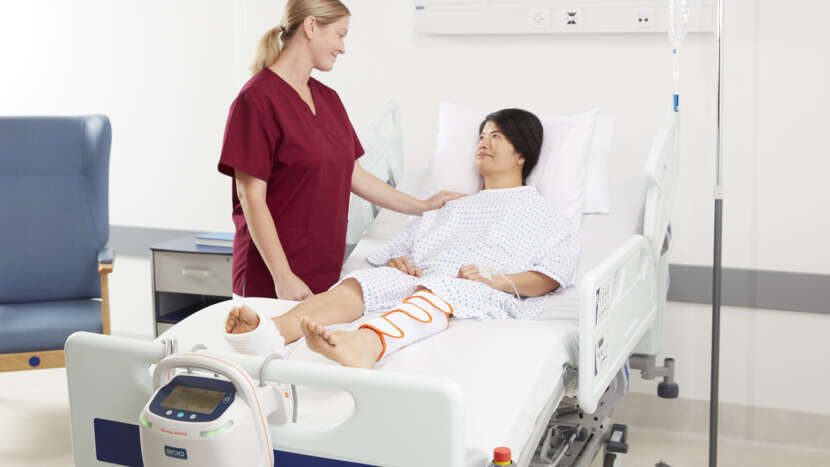
Built on a uniform (single-chamber bladder) compression philosophy, Flowtron DVT is an extensive range of foot, calf and thigh garments addressing a wide variety of clinical needs and patient types
COMPLIANCE BEGINS WITH COMFORTWith comfortable, premium fabrics making the patient more inclined to wear the garments during therapy, Flowtron addresses the core challenge of comfort in VTE prevention. Proven comfortable, Flowtron garments promote effective prevention and improved patient outcome.1-2
REFERENCES:
1. Ellis J. The textile properties of Deep Vein Thrombosis (DVT) garments: a factor in patient compliance with Intermittent Pneumatic Compression (IPC) systems. Arjo Whitepaper. March 2019. Arjo.A00096.1.0.INT.EN.
2. Pagella P, Cipolle M, Sacco E et al. A randomised trial to evaluate compliance in terms of patient comfort and satisfaction of two pneumatic compression devices. Orthop Nurs. 2007; 26(3):169-74.
References
1. Arjo Independent Test Data on File. Tri Pulse: water vapor resistance, thermal resistance (single plate method), drying time, liquid wicking rate and water vapor permeability testing. September 2019. Test report E-008677/C.
2. Kucher N, Koo S, Quiroz R et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005; 352:969-977.
3. Pagella P, Cipolle M, Sacco E et al. A randomised trial to evaluate compliance in terms of patient comfort and satisfaction of two pneumatic compression devices. Orthop Nurs. 2007; 26(3):169-74.
Extensive range of foot, calf and thigh garments addressing a wide variety of clinical needs and patient types
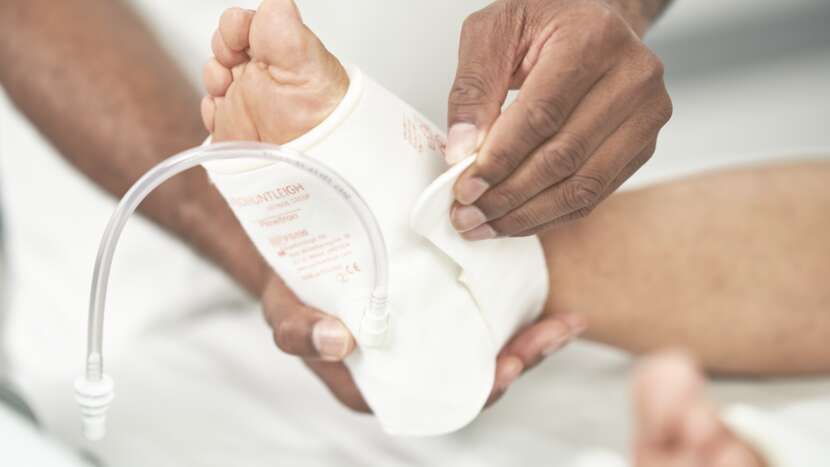
COMFORTABLE FABRIC
Lightweight, breathable fabric helps prevent the build-up of heat and moisture1
VELCRO® FASTENERS
Simple and robust Velcro® closures helping to promote effective therapy by providing a secure and snug fit2
APPLICATION GUIDE
Clear visual instructions printed on the garment for ease of use and safety in application3
NEW CONNECTOR DESIGN
New ergonomically and sustainably designed garment connector promoting caregiver ease of use and patient safety4
REFERENCES:
1. Ellis J. The textile properties of Deep Vein Thrombosis (DVT) garments: a factor in patient compliance with Intermittent Pneumatic Compression (IPC) systems. Arjo Whitepaper. March 2019. Arjo.A00096.1.0.INT.EN.
2. Busby J, Holst K, Hansson K. User feedback on the Flowtron® ACS900 pump and Tri Pulse garment range. Arjo Whitepaper. June 2021. Arjo.A00491.1.0.INT.EN.
3. Arjo Data on File: Formative Evaluation Report 100082820. December 2019.
4. Arjo Data on File: SmartSense 2 Connector Design Report 100127048. Memo to File. November 2021.
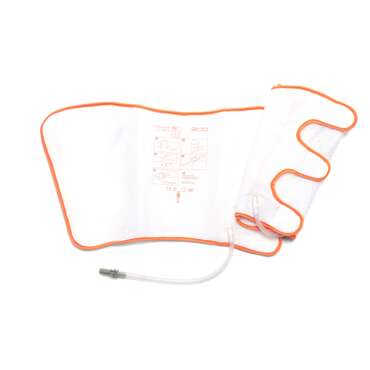
DVT calf and calf & thigh garments are available in a variety of sizes ranging from standard to bariatric. The foot garments are also available in 2 sizes.
| Usage | |
|---|---|
| Calf and calf & thigh garments inflate at a pressure at 40 mmHg with a cycle time of 12 seconds inflate and 48 seconds deflate. | . |
| Foot garments inflate at a pressure at 130 mmHg with a cycle time of 3 seconds inflate and 27 seconds deflate x 2 per inflation cycle. | . |
| Ordering Information | |
| Code | Product Description | Quantity per Carton |
| DVT5 | Small Calf Garment(up to 360 mm/14" calf circumference) | 10 pairs |
| DVT10 | Standard Calf Garment*(up to 430 mm/17" calf circumference) | 10 pairs |
| DVT20 | Large Calf Garment(up to 580 mm/23" calf circumference) | 10 pairs |
| DVT30 | Standard Thigh Garment*(up to 710 mm/28" thigh circumference) | 10 pairs |
| DVT40 | Large Thigh Garment(up to 890 mm/35" thigh circumference) | 10 pairs |
| DVT60L | Extra Large Bariatric Fit™ Calf Garment(up to 810 mm, 32" calf circumference) | 10 pairs |
| FG100 | Regular Foot Garment*(up to shoe size 7 (UK), 40 (Euro)(up to female shoe size 9 (US)(up to male shoe size 7 (US) | 10 singles |
| FG200 | Large Foot Garment*(shoe size 7½ or above (UK), 41 (Euro)(female shoe size 9½ or above (US)(male shoe size 7½ or above (US) | 10 singles |
Explore this product
Type: Brochure
Type: Brochure
Type: Product specifications sheet
Type: Product specifications sheet
Type: Instructions for use (IFU)
Type: Sales support material
Type: Clinical summary and/or focus document
Type: Whitepaper
Type: Whitepaper
Type: Whitepaper
Type: Brochure
Type: Brochure
Type: Product specifications sheet
Type: Product specifications sheet
Type: Sales support material
Type: Clinical summary and/or focus document
Type: Whitepaper
Type: Whitepaper
Type: Whitepaper
Type: Instructions for use (IFU)