Skin IQ® 365
Reusable powered microclimate managing coverlet
An effective and intuitive solution for the prevention and management of pressure injuries.
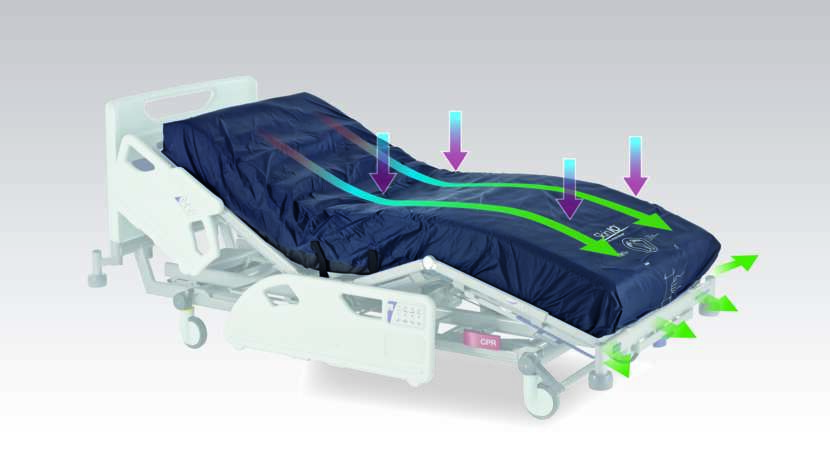
Skin IQ® 365 is a reusable, powered coverlet for use in combination with a pressure redistribution surface to help prevent pressure injuries. Skin IQ uses Negative Airflow Technology® (NAT) to continually draw away excess moisture and help to control temperature at the skin/surface interface (microclimate).Microclimate refers to the conditions of moisture and temperature at the point(s) of skin/surface interface¹, and is a key factor for patients at risk of skin maceration and breakdown. Excess moisture and/or temperature can increase skin sensitivity to the damaging effects of pressure, shear, and friction².
References:
1. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice. Guideline. Emily Haesler (Ed). Cambridge Media: Perth, Australia; 2014.
2. International Review. Pressure Ulcer Prevention: Pressure, Shear, Friction and Microclimate in Context. A Consensus Document. London: Wounds International, 2010.
- Negative airflow technology® (NAT)
continually draws excess moisture away from the skin/mattress interface
Bench studies show that the Skin IQ range of powered microclimate management coverlets remove 3.8x more moisture at the skin/mattress interface¹ than the same mattress without Skin IQ and provide a moisture vapour transfer rate (MVTR) of 170 g/m2/hr¹
- Waterproof, vapour-permeable top layer offers a microbial barrier² and helps to reduce shear and friction³
- Designed for compatibility with most pressure redistribution mattresses on the market today
- Integrated with Atmosair Velaris® and Auralis® Velaris and Auralis pumps feature integrated Skin IQ power ports
- Quick, intuitive setup and maintenance: Simply fit over support surface/mattress and plug in to operate
- Low profile design
1. Arjo Data on File, Skin IQ Reusable System Functional Test – Test Report 100014338 pg. 6
2. Arjo Data on File, Mould Resistence Test – Test Report TWNC01189291.
3. Arjo Data on File, Skin IQ Coefficient of Friction – Test Report 100018362 pg. 1
continually draws excess moisture away from the skin/mattress interface
Bench studies show that the Skin IQ range of powered microclimate management coverlets remove 3.8x more moisture at the skin/mattress interface¹ than the same mattress without Skin IQ and provide a moisture vapour transfer rate (MVTR) of 170 g/m2/hr¹
- Waterproof, vapour-permeable top layer offers a microbial barrier² and helps to reduce shear and friction³
- Designed for compatibility with most pressure redistribution mattresses on the market today
- Integrated with Atmosair Velaris® and Auralis® Velaris and Auralis pumps feature integrated Skin IQ power ports
- Quick, intuitive setup and maintenance: Simply fit over support surface/mattress and plug in to operate
- Low profile design
1. Arjo Data on File, Skin IQ Reusable System Functional Test – Test Report 100014338 pg. 6
2. Arjo Data on File, Mould Resistence Test – Test Report TWNC01189291.
3. Arjo Data on File, Skin IQ Coefficient of Friction – Test Report 100018362 pg. 1
| Weight and dimensions | |
|---|---|
| Weight capacity kg | 227 kg |
| Weight capacity lb | 500 lb |
| Overall Length mm | 2134 mm |
| Overall Length in | 84 in |
| Overall Width mm | 914,4 mm |
| Overall Width in | 36 in |
| Depth/Height of surface mm | 178 mm |
| Depth/Height of surface in | 7 in |
| Coverlet Height mm | 6,35 mm |
| Coverlet Height in | 0,25 in |
| Packaging | Single unit |
| Electrical | |
| Voltage | 100 - 240 VAC |
| Frequency | 50 - 60 Hz |
| Ampere Rating | 0.5A |
| Power Supply | DCV, Reusable and cord |
| Operating Temperature Range C | 14 - 35 °C |
| Operating Temperature Range F | 57.2 - 95 °F |
| Storage Temperature Range C | -29 - 60 °C |
| Storage Temperature Range F | 20.2 - 140 °F |
| Classification | B |
| Standards | IEC60601-1:2006 |
Explore this product
Filter
Filter
PIP International Guidelines
Type: Brochure
Skin IQ Brochure
Type: Brochure
Skin IQ Guidelines 12 Page
Type: Brochure
Skin IQ Solution Guide
Type: Brochure
Skin IQ 365 Instructions for Use
Type: Instructions for use (IFU)
Keeping Cool under Pressure Poster
Type: Clinical summary and/or focus document
Skin IQ Case Study Brochure
Type: Clinical case study
PIP International Guidelines
Type: Brochure
Skin IQ Brochure
Type: Brochure
Skin IQ Guidelines 12 Page
Type: Brochure
Skin IQ Solution Guide
Type: Brochure
Keeping Cool under Pressure Poster
Type: Clinical summary and/or focus document
Skin IQ Case Study Brochure
Type: Clinical case study
Skin IQ 365 Instructions for Use
Type: Instructions for use (IFU)