Clinical Insights: Voice of customer for the Flowtron ACS900 pump & Tri Pulse sequential garments
 Today, we welcome Grace Hukushi, RN, BSN, Arjo Clinical Manager, to discuss voice of customer research conducted with clinical end-users of the Flowtron ACS900 pump and Tri Pulse sequential garment range in several countries.
Today, we welcome Grace Hukushi, RN, BSN, Arjo Clinical Manager, to discuss voice of customer research conducted with clinical end-users of the Flowtron ACS900 pump and Tri Pulse sequential garment range in several countries.
A formal critical care nurse, she has 20 years of clinical expertise working with our partners in venous thromboembolism (VTE) prevention solutions, clinical educator and trainer, has acted as a clinical contributor for leading Nursing Drug Handbooks, and has co-authored articles related to VTE prevention, among others.
Can you explain why VTE preventative measures are important for those at risk?
VTE prophylaxis has been identified as one of the top safety practices to prioritize to improve patient safety in hospitals. Research has shown that approximately 70% of cases of hospital-acquired VTE are preventable through the use of thromboprophylaxis measures. Most hospitalized patients have at least one risk factor and the majority of VTE events are related to hospitalization (hospital-acquired) where clinically effective prophylaxis is readily available. Though highly preventable in many cases, this condition is non-discriminatory as it may affect anyone, may result in long-term complications, and may be potentially fatal. VTE is associated with significant patient morbidity and mortality, and is the most common, preventable cause of inpatient deaths.1
Mechanical prophylaxis systems, commonly used in hospitals, are indicated for the prevention of DVT in patients at risk. Can you elaborate on the advantages of intermittent pneumatic compression (IPC) systems?
There are many advantages of IPC devices documented in the literature including: safe, noninvasive, cost effective whereas prevention is less costly than treatment, there are no inherent bleeding risks, and are clinically-effective in reducing the risk of DVT in at-risk patient populations. The use of IPC provides one aspect of a prevention strategy that can be used either as a standalone therapy, or combined with other methods of prophylaxis, and is a well-established therapy to improve clinical outcomes.
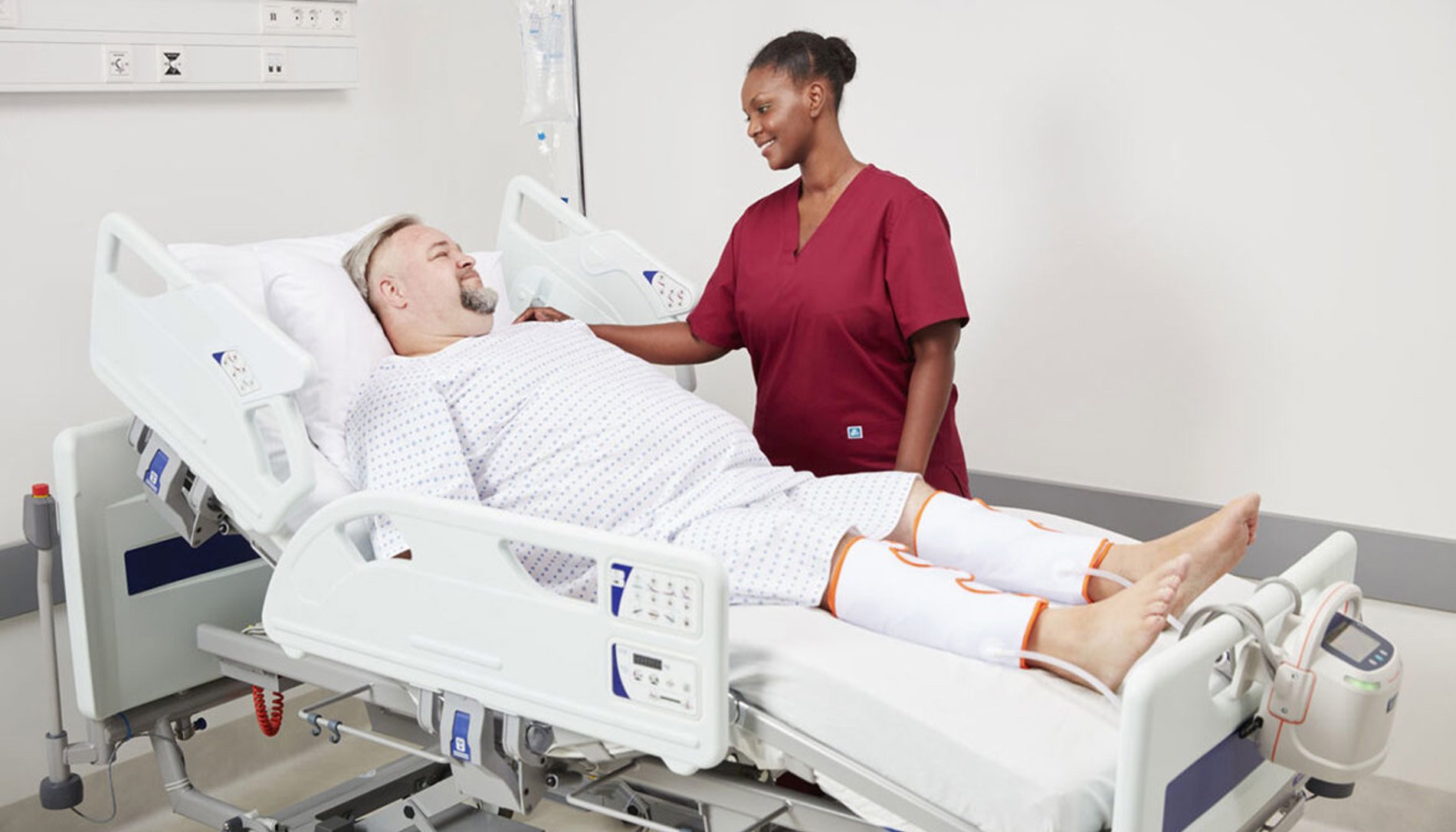
What was the purpose of eliciting voice of customer feedback on your Flowtron ACS900 pump and Tri Pulse sequential garment range?
We consistently listen to our partners and value their feedback. It’s vital to capture their feedback to gain an in-depth understanding about their real-world experiences and insight into our products, and also assists in identifying any potential areas for improvement.
What areas of focus were included in the voice of customer feedback?
Arjo captured clinical end-user data from 32 hospitals on an international scale via interviews and surveys in the US, UK, Australia and France. The important focus areas in our voice of customer research using the Flowtron ACS900 and Tri Pulse sequential garments are all critical components required to improve clinical care and included:
- Patient compliance
- Patient comfort
- Ease of use
- Safety
What were some of the findings in the voice of customer interviews?
All findings captured from clinical end-users around the world were overwhelming positive in all areas of focus and provided substantial insight into the overall experiences and perceptions of our partners using our VTE prevention clinical solutions. Almost 98% of clinical end-users indicated that they would highly recommend the Flowtron ACS900 with Tri Pulse sequential garments to other facilities and caregivers/clinicians!
Where can I learn more about the voice of customer clinical end-user feedback results and the Arjo Flowtron ACS900 and Tri Pulse sequential garment range?
Visit Arjo for more information and download our Flowtron ACS900 VTE Prevention Solutions Brochure and “User Feedback: Flowtron ACS900 Pump and Tri Pulse Garment Range” white paper to learn more about clinically-effective VTE prevention solutions to improve clinical outcomes and quality patient care.
Reference:
- Centers for Disease Control and Prevention. Data and Statistics on HA-VTE. https://www.cdc.gov/ncbddd/dvt/ha-vte-data.html. (Accessed online August 26, 2021).