‘Microclimate Does Matter’ – Analysis of RCT-based Results for At-Risk Patients
(-EUR ING David NewtonA,C M.Eng, C.Eng, MIET, MIEEE, Sara TacksonB,C PT, MPT, CWS)
A pressure injury (PI) results in adverse clinical impact for patients and staff along with increased financial burden to the healthcare facility, potential litigation costs1, and additional federal reporting implications2

Background
A pressure injury (PI) results in adverse clinical impact for patients and staff along with increased financial burden to the healthcare facility, potential litigation costs1, and additional federal reporting implications2.
Implementing the right solution at the right time for the patient is a well-established clinical option for the prevention and treatment of PI’s3. The application of a microclimate coverlet onto an appropriate pressure redistribution surface can be used to address the patient’s microclimate needs while also reducing associated friction and shear forces4.
Purpose
Our analysis provides interpretation of the publicly reported resultsD,5 of a large randomized control trial (RCT) which investigated the effectiveness of microclimate management (MCM) features of support surfaces for PI prevention5.
The RCT involved known ‘at-risk’ patients for PI with clinically identified microclimate conditions (e.g. Braden moisture sub-scale 1 or 2) and were recruited at a single academic medical center between September 2018 and April 2022.
Methodology
This analysis specifically focuses on 2 of the original 7 trial groups within the RCT due to particularly interesting results of PI’s within the RCT and so invited further investigation.
The patients were randomized into 7 trial groups using a different support surface, with each group consisting of around 64 patients. The support surface used for each group was blinded to the RCT principal investigator and anonymized in the study publicity to date. Each support surface was described by a letter and a short description based on their construction properties.
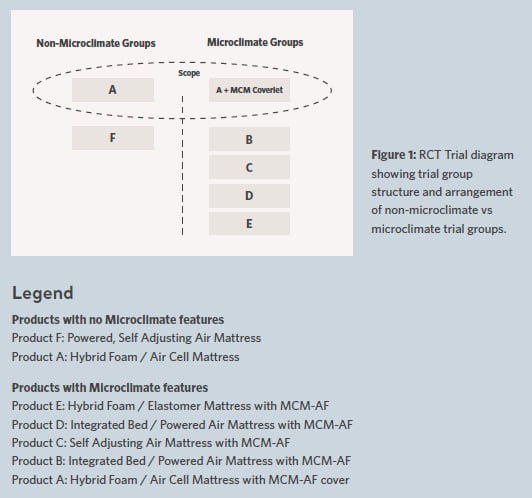
The RCT consisted of 2 distinct groupings as seen in Figure 1.
One grouping consisted of 2 full body support surfaces without any microclimate management feature providing a non-microclimate reference within the study. The other grouping included 5 different full body support surfaces which specifically claimed microclimate features. These included different features / characteristics providing microclimate performance to various levels but were described generally as having a ‘low air loss’ or ‘microclimate management using airflow (MCM-AF)’ feature.
During the patient stay and as part of the RCT all PI’s were recorded and staged according to the National Pressure Injury Advisory Panel (NPIAP) guidelines. The RCT results reported PI’s at Stage 1, Stage 2, and a combined section including Stage 3 and Deep Tissue Injury (DTI).
Also part of the RCT, various test methods from the US National Standard SS-1:20196 (which was developed by NPIAP/S3I) were used to independently assess each product’s microclimate performance using Section 3:Body Analog, Section 4:Sweating Guarded Hot Plate & Section 8:Heated Water BladderA comparison of patient outcomes in each trial group in terms of PI incidence and severity was performed and an attempt to correlate this with the measured microclimate performance of each associated support surface.
Our analysis of the RCT results focuses specifically on 2 of the 7 original support surface trial groups described as ’Product A’ & ‘Product A with MCM-AF cover’. These 2 trial groups provided a unique comparison within the wider trial structure since they share the same underlying support surface (i.e. Product A). Therefore, the only difference between these two groups was the addition of the microclimate coverlet providing microclimate and shear mitigation.
The RCT investigator reported no statistical significance for the PI differences between any of the 7 groups.
Product microclimate performance across the support surfaces was measured and the results are shown in Figures 5–8.
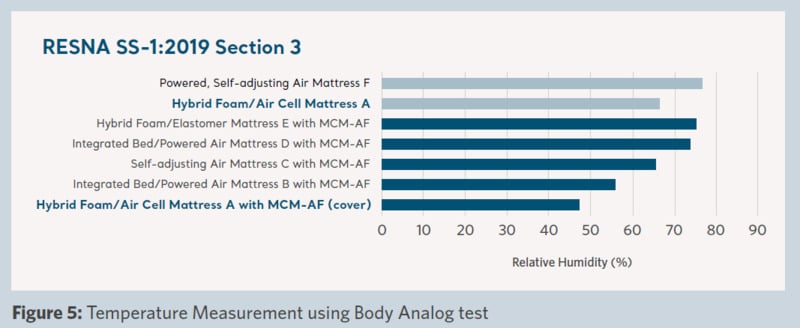
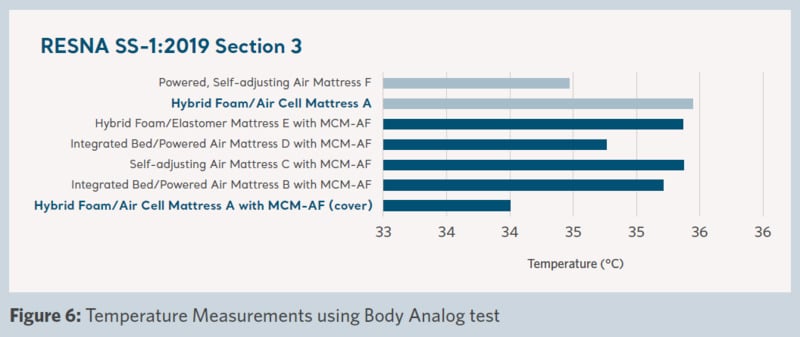
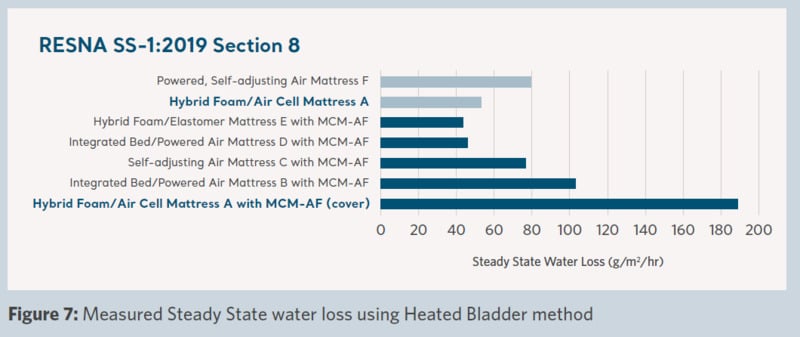
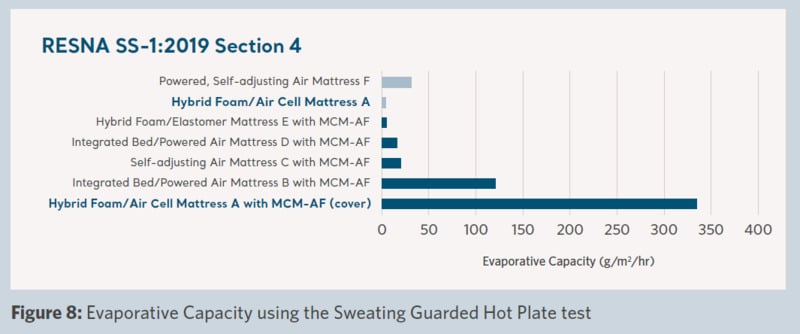
Figure 5 shows the significantly lower level of humidity (moisture) that was measured during the test with ‘Product A with MCM-AF cover’ than ‘Product A’ on its own without the coverlet. In addition, it was lower compared to any other support surface in the trial.
Figure 6 shows the interface temperature measured during the test. A lower temperature was achieved with the use of the ‘Product A with MCM-AF cover‘ compared to ‘Product A’ without the cover. In addition, a lower temperature was achieved than all the other support surfaces in the trial.
Figure 7 shows the measured water removal characteristics of the surface. ‘Product A with MCM-AF cover‘ has a considerably higher water removal capability than ‘Product A’ and all other products in the trial. This demonstrates the removal of moisture from the patient's environment and can result in the skin being maintained in a lower moisture condition.
Figure 8 shows the evaporative capacity of the products in the trial, which is an estimate of the capability of moisture removal of a full-body support surface. The high level of evaporative capacity demonstrated by the ‘Product A with MCM-AF cover’ correlates directly with the measured moisture removal test shown in Figure 7 and is significantly larger than any of the products in the trial.
Discussion
The RCT PI outcomes data presented in Figure 4 an effect that could be due to the addition of the microclimate coverlet to a support surface. An improvement in patient PI outcomes both in number and their stage/severity was demonstrably achieved with the use of the microclimate coverlet compared to those that occurred with the same support surface without the coverlet. This is of particular relevance as it is associated with the primary investigative hypothesis of the RCT, hence these results warranted further specific analysis and investigation by the authors.
The two trial groups of ‘Product A’ & ‘Product A with MCM-AF cover’ differed only by the addition of a coverlet designed for microclimate control. They shared the same underlying reactive support surface and therefore can be expected to have the same pressure redistribution characteristics, meaning the only technical difference between the two groups was microclimate management.
It was reported by the RCT that the patient characteristics including age, sex, race, ethnicity, Body Mass Index (BMI), and overall Braden score were noted as being comparable across the individual trial groups.
The difference in the PI outcomes was reported by the RCT as not being statistically significant. This was due to the limitations in the powering of the RCT, together with the resulting low PI incidence rate. It therefore meant that it was not possible to prove that the differences between groups were statistically significant or they were the result of any particular surface performance parameter.
Analyzing the PI rates of the trial groups (Figure 4) shows the outcome ranking of ‘Product A’ alone was higher than the overall trial average but ‘Product A with MCMAF cover’ was lower than the trial average. This notable difference also has investigative interest, hence further analysis is suggested.
The appreciable difference in PI outcomes appears consistent with the difference in measured microclimate performance between ‘Product A’ and ‘Product A with the MCM-AF cover’. It is postulated by the authors of this poster, that the addition of the microclimate coverlet was directly responsible for the outcome difference in overall PI incidence and in PI severity by helping to address both the direct microclimate effects and the associated microclimate-related shear and frictional effects4. It could be suggested that the demonstrable higher level of microclimate performance provided by the microclimate coverlet appears to mitigate some of the microclimate-related risk and causal factors in PI formation. Further investigative study is warranted to confirm this assertion.
It is generally known that higher temperature increases the permeability of the stratum corneum leading to increased stratum corneum hydration. Higher levels of stratum corneum hydration increases the friction between skin and any materials in contact, enhances absorption of irritants, decreases skin stiffness, and reduces skin strength. All of these effects are understood to lead to greater likelihood of PI development and higher PI severity.
In the two study groups, there was a difference of 3 PI’s between the two subgroups of ‘Product A’ & ‘Product A with MCM-AF cover’. Also there was a significant reduction in the severity of the PI’s that were present in each group which provides important benefits to patient health and can reduce care requirements together with associated costs. PI cost has been reported between $20,900 – $151,700 per event dependent upon severity1. The difference of 3 PI’s could equate to a significant potential variance in resulting PI cost between the mentioned groups by $60,000 – $450,000. The lower end of this estimate is significantly more than the cost of providing the microclimate coverlet as a preventive measurement for the entire group. This means that it is financially justified to upgrade existing support surfaces with the addition of a microclimate coverlet for at-risk patients with identified microclimate risk factors (e.g. Braden sub-score 1 or 2).
There were a number of known limitations to the RCT.
Firstly, the duration of the study (2018-2022) spanned the COVID-19 pandemic, as a result this could have impacted nursing care during part of the study timeline. It is reasonable to expect that the effects of the pandemic in the facility in terms of staffing levels, reduced patient contact time and more critical priorities could have had some impact and hence effect.
Secondly, all support surfaces used in the trial were operated in a reactive mode of operation to provide a consistent comparison between individual products. It is possible that the use of other modes of support surface operation may have been more suitable for some or all of the at-risk patients in the trial in terms of pressure redistribution. For example, using an active mode support surface (alternating pressure) may have resulted in different PI outcomes and product performance measurements than those reported using a reactive mode. This aspect is perhaps something to be considered when planning any future RCT’s or attempts at replication of this RCT.
Conclusion
Our analysis of the outcomes of the 2 trial groups indicates the use of a microclimate coverlet can provide effective microclimate capabilities to augment the operation of a standalone support surface.
An important set of data associated with the microclimate performance of support surfaces was revealed in the RCT. The microclimate coverlet provides a continuous high level of microclimate management across the entire body as demonstrated by the RCT testing of the product. This concurs with previous testing of this aspect that has been performed and reported4.
The use of a microclimate coverlet for at-risk patients with moisture challenges flagged in their risk assessment subscale, may provide a fiscally responsible pathway to mitigate the persistent PI problem in healthcare today. As a targeted approach on a patient by patient basis, the coverlet can be deployed to augment an existing support surface and other clinical interventions such as manual repositioning.
We conclude that further detailed analysis of the RCT results specifically in relation to these two sub-groups is clearly warranted to provide confirmation of the beneficial effect of the addition of the microclimate coverlet.
Download the document
Get personalized advice and solutions
References
- AHRQ webpage on pressure injuries, www.ahrq.gov/patient-safety/settings/hospital/resource/pressureulcer/tool/pu1.html accessed 04/October/2024.
- PSI Technical Documentation, Version v2024, Agency for Healthcare Research and Quality, Rockville, MD. https://qualityindicators.ahrq.gov/measures/psi_resources. Accessed October 3rd, 2024.
- Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline, The International Guideline. Emily Haesler (ed), EPUAP/NPIAP/PPPIA:2019.
- ‘Getting Stressed by Shear?’ – The linkage between Microclimate and Shear, Newton D & Tackson S, NPIAP2024 poster, accessible via Arjo reference A00703.1.0.INT.EN.
- Clinicaltrials.gov file entry for NCT03351049 accessed 10th January 2025.
- Requirements and Test Methods for Full Body Support Surface SS-1:2019, ANSI/RESNA.
Acknowledgments
- Advanced Development Manager at Arjo Inc.
- Clinical Consultant at Arjo Inc.
- Contributing members of NPIAP/S3I.
- RCT data courtesy of Dr Brienza, University of Pittsburgh as presented at WOCN2023 conference. Images adapted with kind permission of Dr Brienza.
- Product A = Arjo AtmosAir9000 Support Surface.
- MCM-AF cover = Arjo SkinIQ Microclimate Manager.