Understanding the Importance of Compression Therapy in VTE Prevention
When we get a small wound, we often praise our blood’s ability to clot to protect the wound and prevent further blood loss. However, when blood starts to clot in the wrong place at the wrong time, the consequences can be life-changing, even fatal. Every year, blood clots in the deep veins cause extensive suffering for millions of people. In this article we’re taking an in-depth look at blood clots, their causes and how the Flowtron Active Compression System can help prevent the condition.
“There are an estimated ten million cases of Venous Thromboembolism (VTE) per year worldwide. It is a life-threatening condition that can have a significant cost burden on acute care providers and healthcare systems,” says Henrik Runnerström, Global Director in Product Category Management, VTE Prevention at Arjo.
Around 30 percent of patients will die within 30 days of developing VTE, while 25 percent of unexpected inpatient deaths are diagnosed with pulmonary embolism at autopsy1.
Around one third of patients with deep vein thrombosis (DVT) develop post-thrombotic syndrome, a condition causing suffering, swelling and pain1. For 25 percent of these patients2, the resultant chronic ulceration is associated with substantial on-going treatment cost3.
Henrik continues: “In addition to all the human suffering, the high number of VTE cases translates into a significant cost burden for acute care providers and healthcare systems.”
What is VTE?
Venous Thromboembolism (VTE) is the collective term that includes deep vein thrombosis (DVT) as well as pulmonary embolism (PE). DVT is when a blood clot forms in a deep vein, most commonly in the leg, while a PE may occur if the clot breaks free and travels to the lungs, blocking some or all of the blood supply.
Pulmonary embolism is a potentially fatal condition4. Whilst early diagnosis and treatment of VTE may lead to recovery, long-term complications can result in lifelong treatment and suffering.
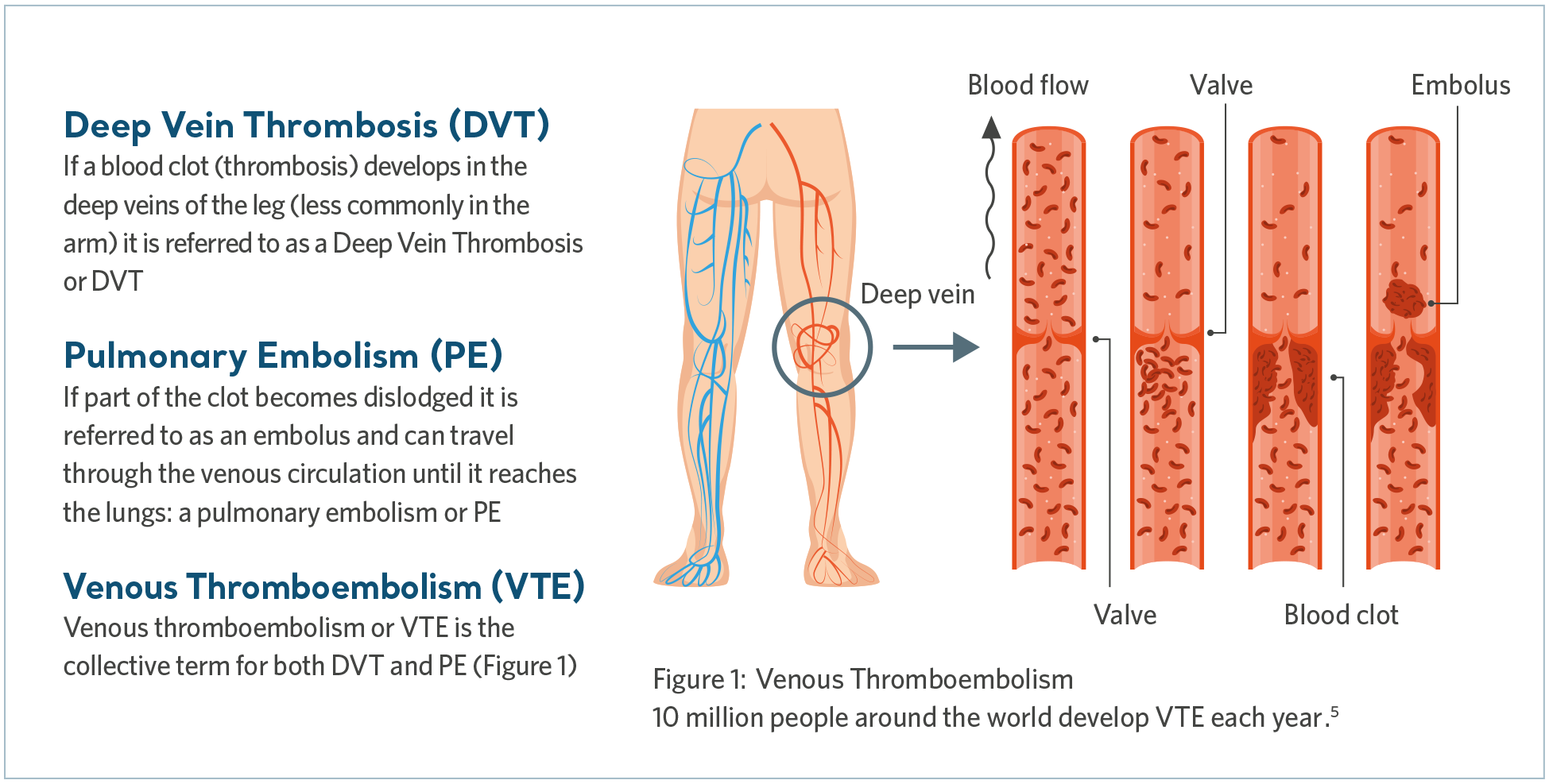
What causes VTE?
VTE is triggered when multiple risk factors, collectively described by ‘Virchow’s Triad’, tip the balance of homeostasis leading to clot formation.6 Let’s look at each element that make up Virchow’s Triad.
Virchow's Triad
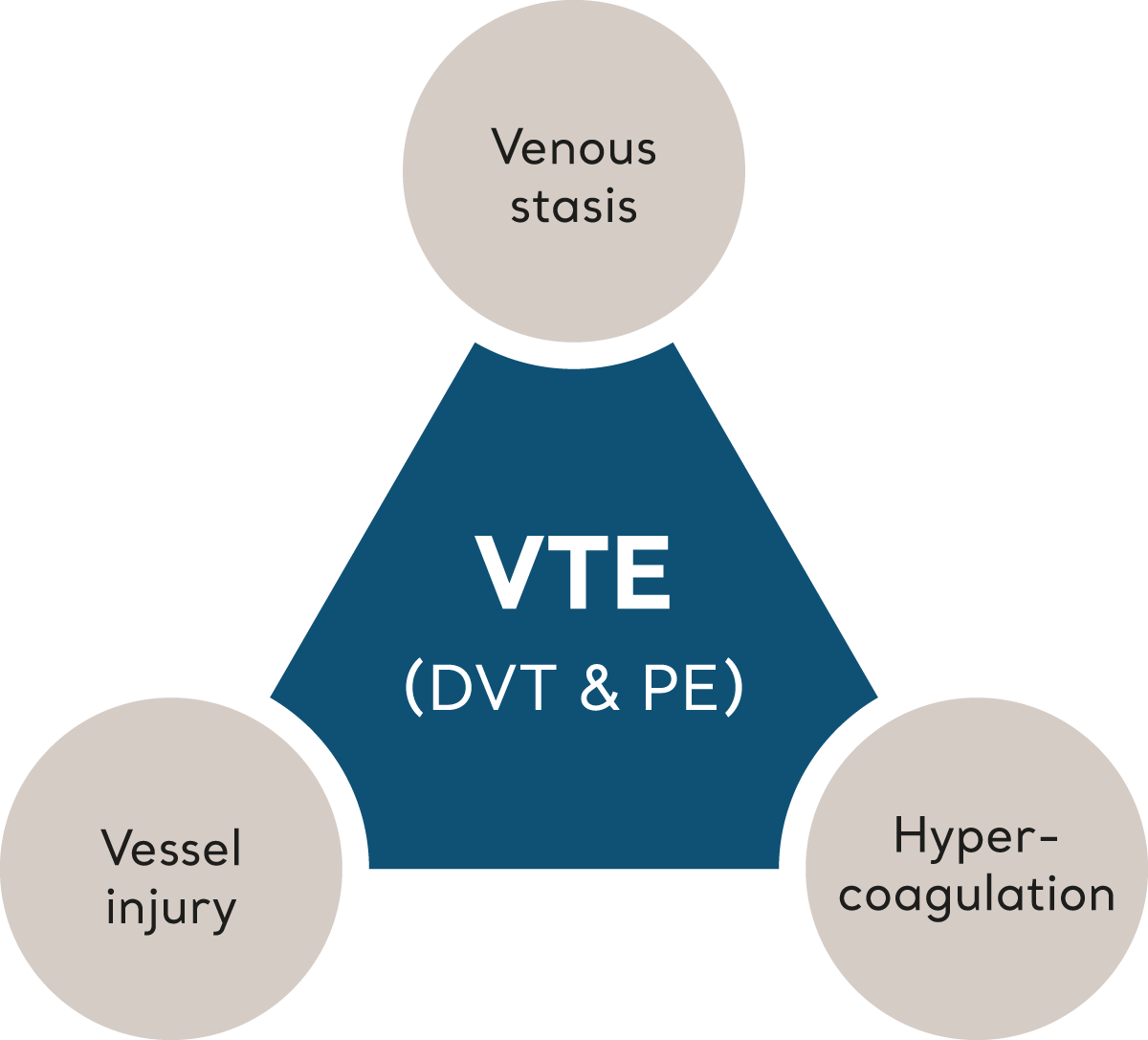
Venous stasis
Circumstances that cause the blood flow to slow (stasis) in the deep veins will increase the risk of VTE. Immobility, perhaps due to age, frailty or prescribed bed rest, is a clear risk factor.
Physical obstruction of the deep vessels, either due to external forces or pressure from tumors and lymph nodes, is also implicated, as are previous DVTs.
Hypercoagulation
Changes in blood density and chemistry can increase the tendency for the blood to clot. This is associated with conditions such as simple dehydration to hypoxia, malignancy, trauma, hormone therapy, systemic inflammatory disease and genetic predisposition.
Vessel Injury
Injury may occur through accidental trauma or through medical interventions such as surgical or invasive procedures. Once injury occurs, a normal physiological series of events initiate platelet adhesion and the eventual formation of a blood clot; this protective mechanism seals the damage and begins the healing process. Sometimes however this process is exaggerated or inappropriate.
Who is at risk for VTE?
Surgical inpatients are a particular risk group7 as surgery itself causes the greatest increase in risk, because of the anesthetic (hypercoagulability), muscle relaxants (stasis) and surgical intervention
(vessel damage). It is also clear that non-surgical hospital patients are at high risk of VTE.
Henrik Runnerström says, “There are numerous factors contributing to VTE, and even if the condition is often associated with immobile people and the elderly, it can also happen to a seemingly healthy, fit and athletic individuals.”
Former elite hockey player Stefan Elvenes in Sweden is an example of this. A few months after he retired from ice hockey – following a 22-year career at the highest level in Sweden and Denmark – he noticed a swelling at the back of his left leg.
“When the doctor told me it was a blood clot, I couldn’t believe it. I was only 37 and still very fit. I wasn’t supposed to get something like that,” Stefan says.
After being told that younger and athletic people also can get blood clots, Stefan was treated successfully with blood thinners. However, three weeks after he stopped taking his medicine, he got a severe panic attack.
“In the back of my mind, stopping my medication translated into a risk of getting another blood clot. That probably triggered my panic attacks,” Stefan says. “I was a mental wreck for a long time. But regular therapy at a psychologist helped me snap out of the fears. Today, I have made peace with it.”
Preventing VTE
Pharmacological prophylaxis like the blood thinners Stefan was treated with is one way to deal with VTE. Preventive strategies also involve various mechanical methods, such as Intermittent Pneumatic Compression (IPC) and passive Graduated Compression Stockings (GCS).
IPC is a very well established and proven intervention with a convincing evidence base and few side effects – and it is indicated for use across a wide range of hospitalized patients at risk of VTE.
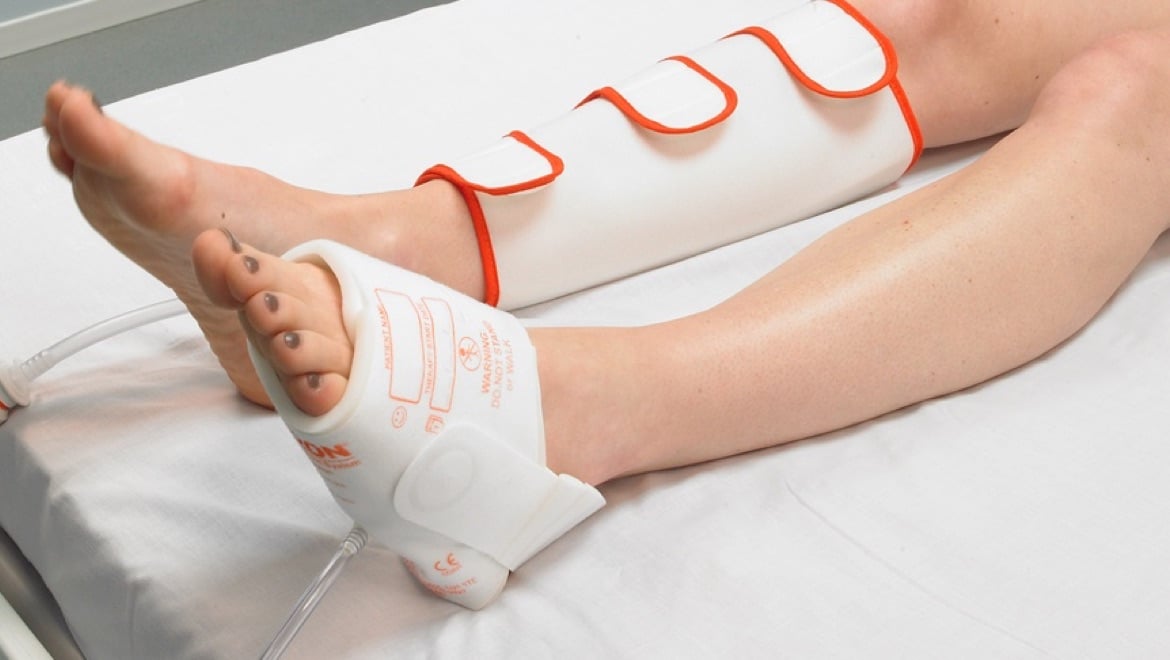
The purpose of IPC is to propel blood from the deeper veins through the intermittent inflation and deflation of a garment connected to an electric pump.
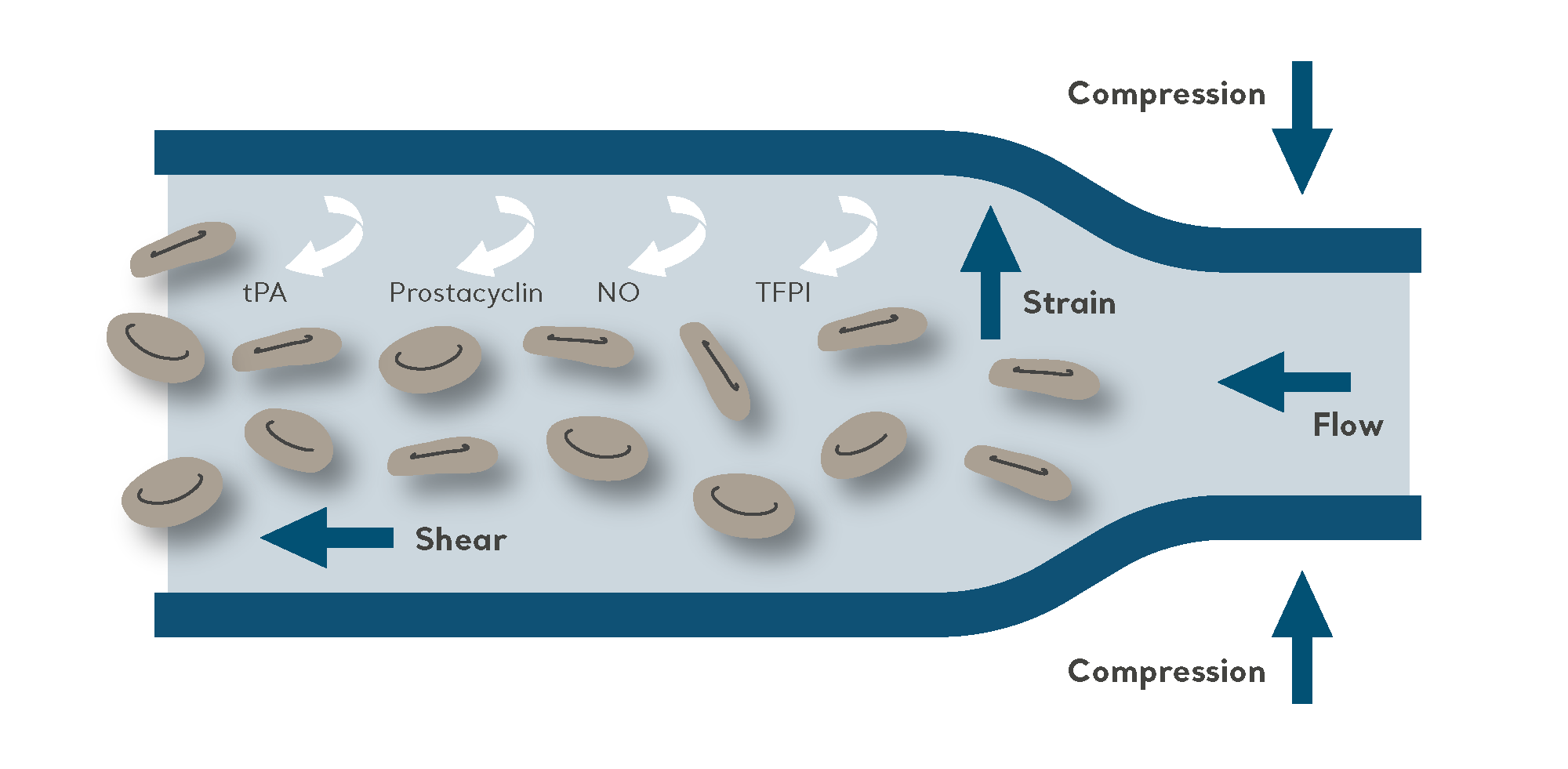
As a non-invasive mechanical method of VTE prophylaxis, IPC is effective when used either as a sole prevention modality, such as for patients at high risk of bleeding. It is also very commonly used in combination with pharmacological prophylaxis for high-risk patients (to further reduce the risk of VTE).
A trusted partner for using IPC in the VTE prevention area
Since the 1970’s, VTE studies clearly demonstrated the protective benefit associated with IPC of the deep veins of the leg. Since then, Arjo has been instrumental in developing easy to use, clinically effective IPC systems to support facilities across the globe. The Flowtron Active Compression System (ACS) range is designed to address clinical efficacy through comfort, ease-of-use and cost-effectiveness.
Arjo is also dedicated to improving the quality of care for patients and helping healthcare providers fight deep vein thrombosis and pulmonary embolism.
“Our commitment goes beyond supplying pumps and garments included in our Flowtron Active Compression System. We want to differentiate Arjo from the competition by being a partner that adds services and valuable knowledge to our offering,” says Henrik Runnerström.
He continues, “Even if healthcare providers are aware of the risks, they may lack the time, training and resources needed to optimize their prevention strategies. Understanding these challenges is the foundation for developing our services and training programs, which are designed to boost the ability to prevent and treat VTE.”
The Flowtron Active Compression System – a true plug-and-play solution
Arjo’s solution, the Flowtron Active Compression System, uses a pneumatic pump to inflate garments around the foot, calf, thigh, or a combination of the three.
Henrik says, “By mimicking the action of the calf muscle pump, IPC increases blood circulation in the deep veins and helps prevent the blood from clotting. It is a well-established and proven therapy with a convincing evidence base.”

The Flowtron ACS900 pump (image above) offers both uniform and sequential compression via a variety of garment types. The SmartSenseTM Automatic Garment Recognition (image below), together with one-button start, make Flowtron a true plug-and-play solution.
“The flexibility reduces the need for multiple pump models in the care facility”, Henrik says. “The system is easy to set up and operate, and the built-in battery enables uninterrupted therapy also when the pump is not connected to a power outlet.”
He continues: “Together with our range of garments, the system is comfortable for the patient, convenient for the healthcare provider and clinically effective at delivering intermittent pneumatic compression – helping to contribute to improved compliance for all those involved in patient care.”
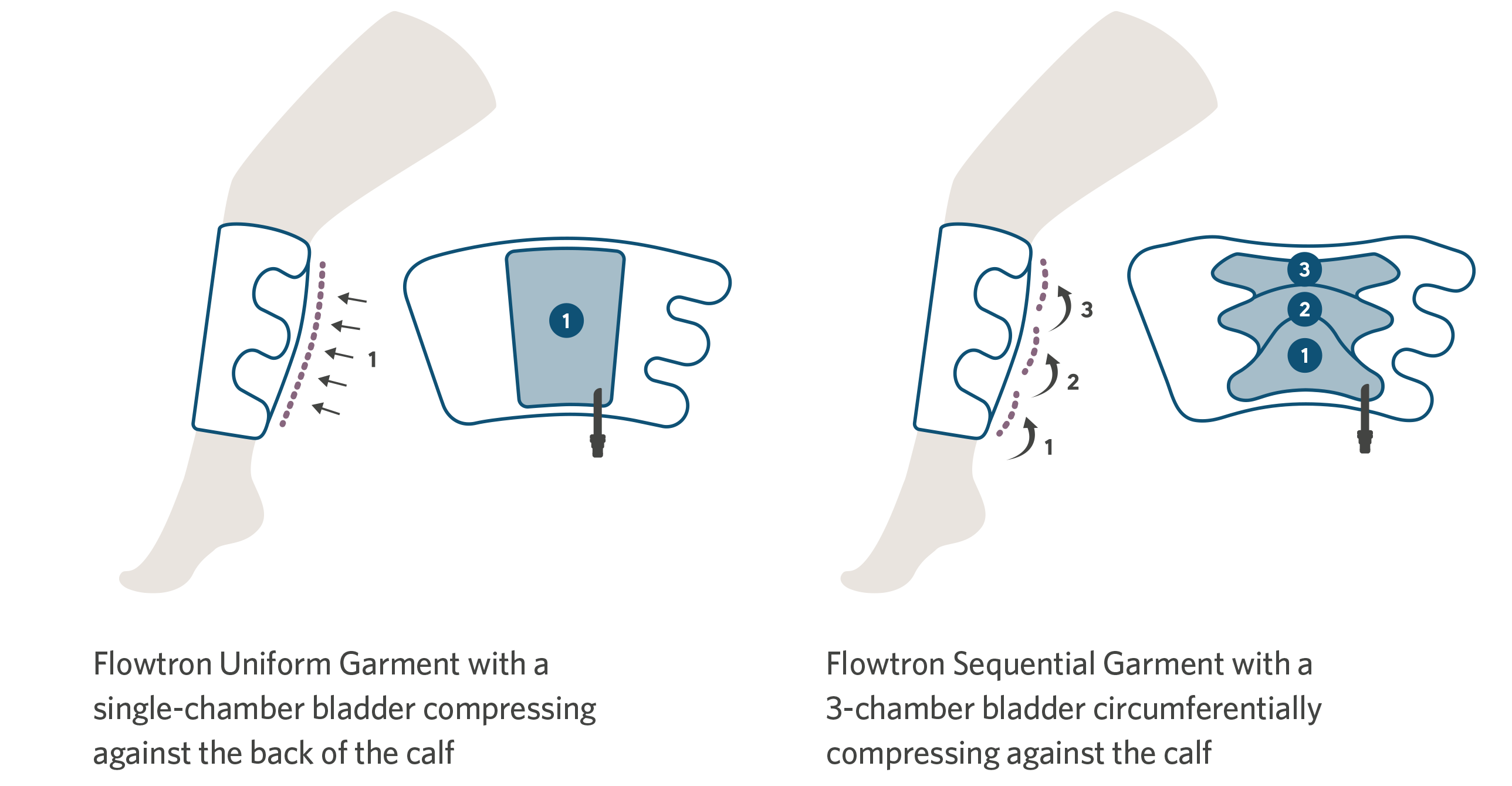
For convenience, there is a choice of Flowtron garments, with calf or thigh-length versions offering either sequential or uniform compression, available in a range of sizes from small through bariatric. Foot compression is available in regular or large foot size. A combination of different garments can be used simultaneously. The choice of garment depends simply upon the preference of the clinician.
Henrik Runnerström explains, “International guidelines recommend therapy to take place continuously for 18-24 hours per day8. Studies show a direct connection between the comfort level and the patients’ willingness to wear garments for longer periods9. We aim to improve patient comfort by using breathable fabrics, which help to keep the patient dry and cool by allowing heat and moisture to pass through.”
Learn more about the Flowtron Active Compression System here
Flowtron IPC – clinical data
Laboratory studies
Many of the early investigative studies that underpin Intermittent Pneumatic Compression as a generic form of VTE prophylaxis, were conducted using predecessors to the contemporary Flowtron range. Although the technology has been updated, the underlying design principles such as cycle pressure, inflation rate and cycle intervals, remain the same.
Amongst studies demonstrating the antithrombotic and profibrinolytic effect of IPC, key findings suggest that IPC makes clots less likely to form. In addition, IPC also increases the suppression of clot formation and breaks clots down once they begin to form.7
The key findings in these studies were that IPC makes clots less likely to form, and that it also increases the suppression of clot formation and breaks up clots once they begin to form.
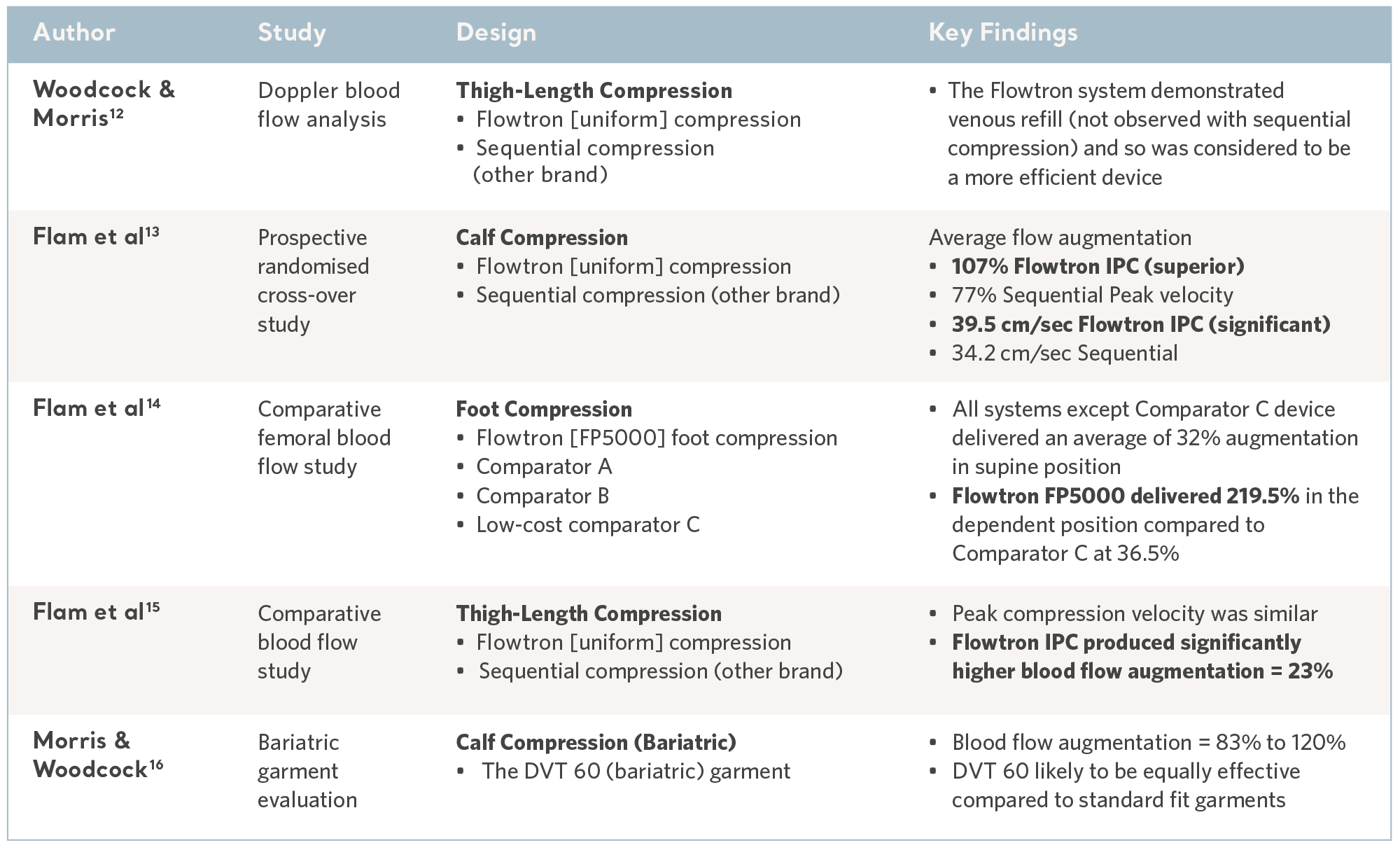
Comparative blood flow studies
The reversal of venous stasis is key to prevention and the Flowtron IPC range has been subject to a number of comparative laboratory tests and consistently demonstrated favorable results10,11. See the table below.
Evidence from the laboratory
Clinical effectiveness
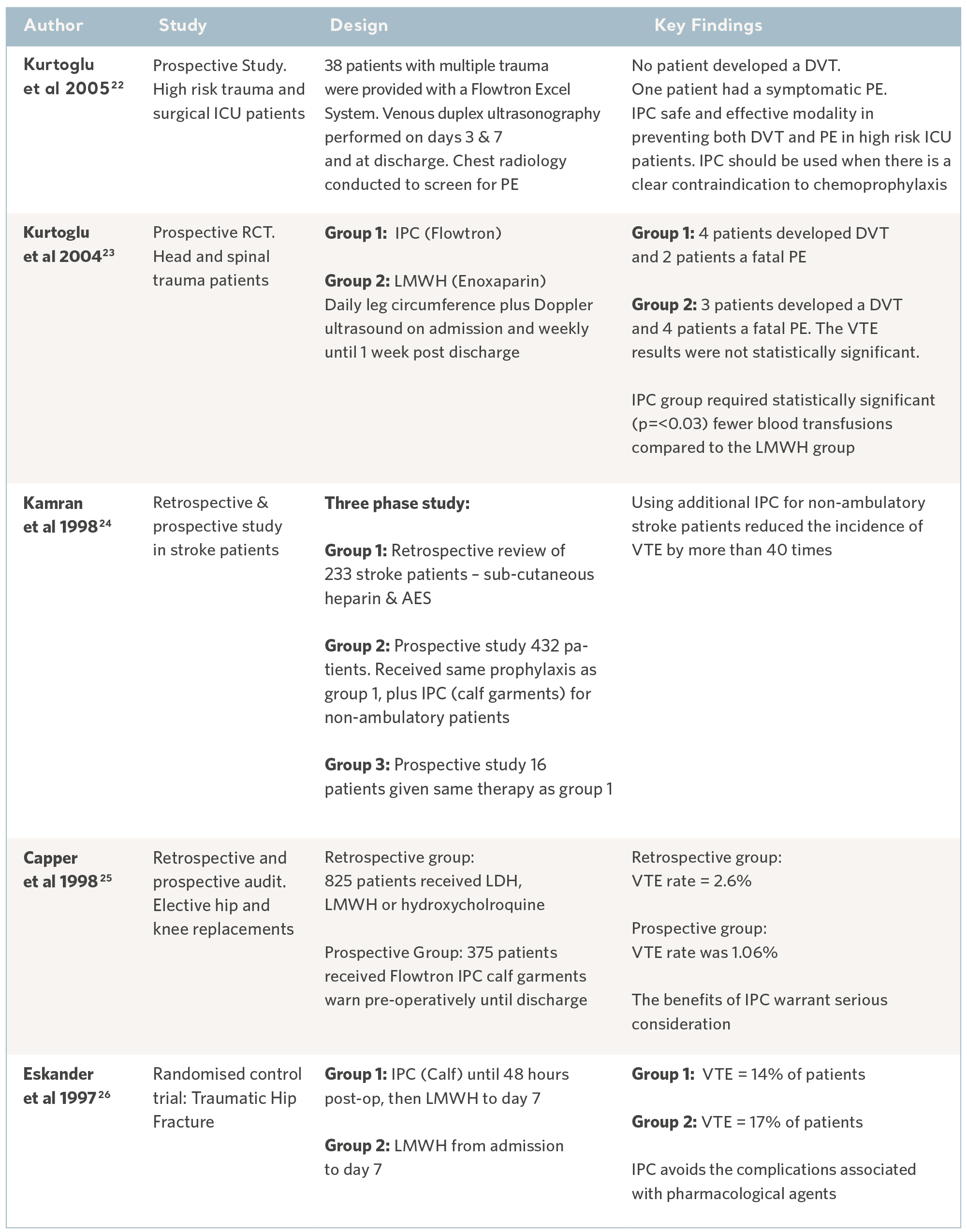
Over the past 30 years, independent specialists have conducted a number of clinical studies. Subjects have been recruited from the highest risk patient populations and across a range of clinical specialties. The results have consistently demonstrated the prophylactic capability of the Flowtron IPC range when used with, or in place of, other methods of prophylaxis. Although IPC is now widely accepted as a valid form of prophylaxis, these legacy studies continue to have value17. See the tables below.
Flowtron Clinical Outcome Studies
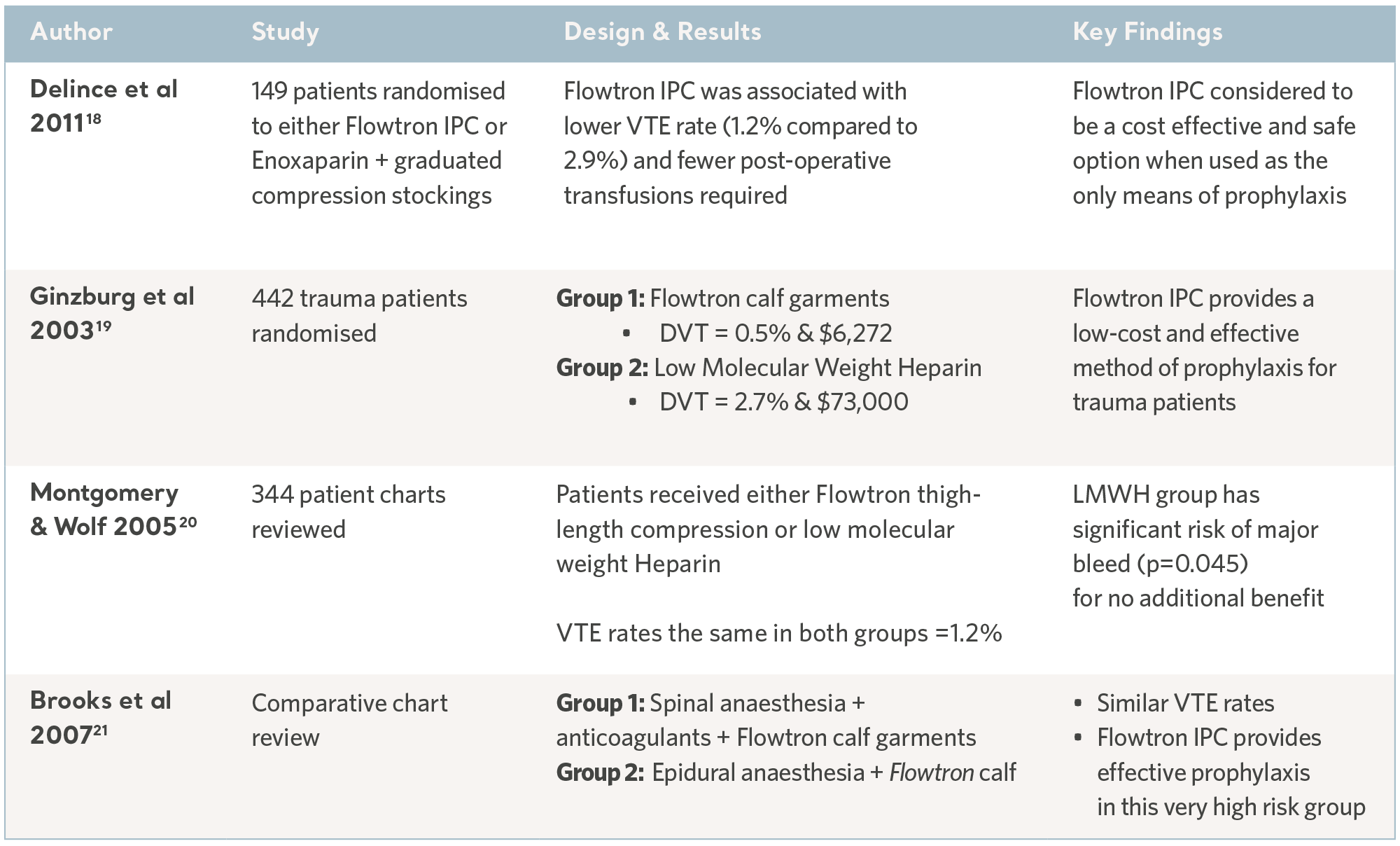
Flowtron evidence in specialty groups
IPC evidence from meta-analysis and systematic reviews
As with all preventative interventions, IPC can only be effective if it is used with the right patient at the right time and that means identifying patients at risk before a VTE incident occurs.
At the same time, the risk of side effects must be considered, in particular the risk of hemorrhage associated with the use of anticoagulation.
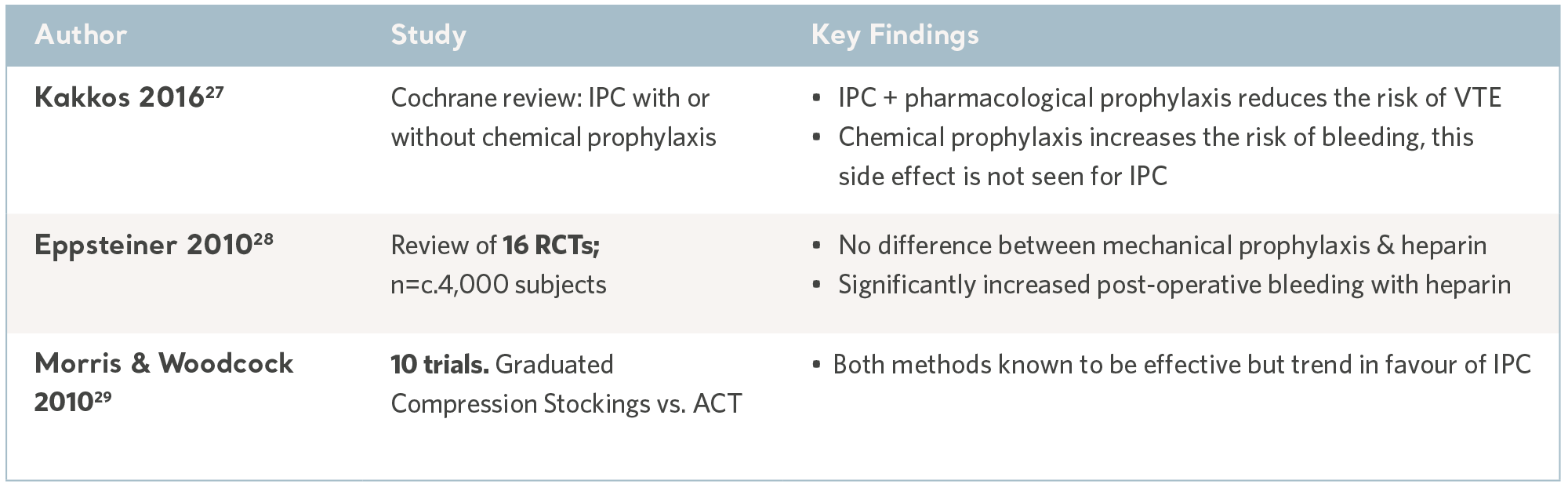
Meta analyses and systematic reviews, where multiple evidence sources are combined to determine the overall clinical utility of IPC, are useful sources of information and can guide prescription.
As an extension of the systematic review, the publication of national and international clinical practice guidelines translate robust and contemporary research into discrete recommendations; knowledge gaps are filled by international consensus panels. While the wording varies, the recommendations are largely consistent and all accept IPC as an effective and safe intervention. See the table below.
IPC Evidence from Systematic Reviews
Convenience
As with any method of prophylaxis, IPC is only beneficial when it is correctly administered and tolerated by the patients who use it; comfort and usability are key considerations and concordance can vary.30
In a busy clinical environment, the device benefits from an intuitive user interface and appropriate safety features to minimize the risk of harm. Some of these aspects have been evaluated in usability trials and through independent technology assessments, these features include safety, quality, ease of use in addition to cost-effectiveness. See the table below.
Evidence from usability studies 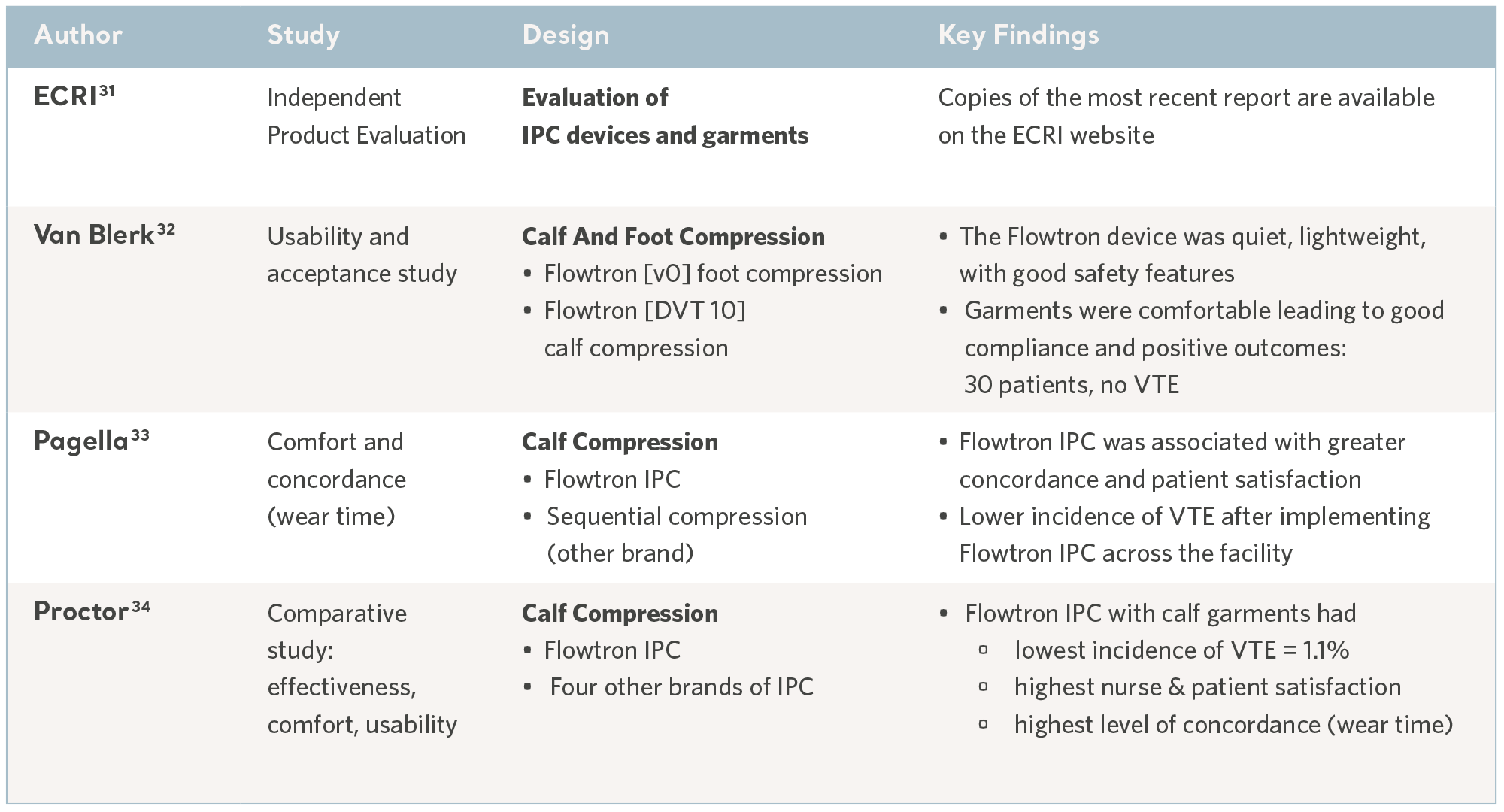
Read more about clinical data regarding Flowtron IPC
References
1- Beckman MG, Hooper WC, Critchley SE et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010; 38(4): S495-501.
2- Nelzen O, Bergqvist D, Lindhagen A. Leg ulcer etiology - a cross sectional population study. J Vasc Surg. 1991; 14: 557-64 cited in Nicolaides A, Fareed J, Kakkar A et al. Prevention and treatment of venous thromboembolism - International Consensus Statement. International Angiology. 2013; 32(2): 111-260.
3- Ruppert A, Steinle T, Lees M. Economic burden of venous thromboembolism: a systematic review. J Med Econ. 2011; 14(1): 65-74
4- Prevention and treatment of venous thromboembolism. Prevention and Treatment of Venous Thromboembolism (VTE) | American Heart Association Last accessed December 2019
5- Jha AK, Larizgoitia I, Audera-Lopez C et al. The global burden of unsafe medical care: analytic modelling of observational studies. BMJ Qual Saf 2013; 22: 809-15.
6- Reitsma PH, Versteeg HH, Middeldorp S. Mechanistic view of risk factors for venous thromboembolism. Arteriosclerosis, thrombosis and vascular biology. 2012; 32(3): 563-8.
7- Nicolaides A, Fareed J, Kakkar A et al. Prevention and treatment of venous thromboembolism - International Consensus Statement. International Angiology. 2013;32(2): 111-260.
8- Guyatt GH, AKL EA, Crowther M et al. Executive Summary: Antithrombotic Therapy and Prevention of Thrombosis. 9th edition. American College of Chest Physicians. Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141(2):7S-47S.
9- Pagella P, Cipolle M, Sacco E et al. A randomised trial to evaluate compliance in terms of patient comfort and satisfaction of two pneumatic compression devices. Orthop Nurs. 2007; 26(3):169-74.
10- Morris RJ, Giddings JC, Ralis HM, et aL. Haematological and haemodynamic comparison of the Kendall AV Impulse™ and the Arjo [Huntleigh] FP5000 Intermittent Pneumatic Foot Compression System. Arjo Clinical Report 2003.
11- Westrich G, Specht LM, Sharrock NE et al. Venous haemodynamics after total knee arthroplasty: evaluation of active dorsal to plantar flexion and several mechanical compression devices. The Journal of Bone & Joint Surgery. 1998; 80B(6): 1057-1066.
12- WoodcockJPandMorrisRJ.TheeffectoftheKendallSCDTMandArjo[Huntleigh]DVT30 garments on femoral and popliteal vein blood flow measurements. Arjo Clinical Report. 2002.
13- Flam E, Berry S, Coyle A et al. Blood flow augmentation of intermittent pneumatic compression systems used for the prevention of deep vein thrombosis prior to surgery. The American Journal of Surgery. 1996; 171(3): 312-315.
14- Flam E, Nackman G, Tarantino D et al. Intermittent pneumatic compression devices of the foot: a comparison of various systems on femoral vein blood flow velocity augmentation in the v supine and dependent, non-weight bearing positions. Arjo Clinical Report 2000.
15- Flam E, Berry S, Coyle A et al. DVT prophylaxis: comparison of two thigh high intermittent pneumatic compression systems. Presented at the meeting of the American College of Surgeons, San Francisco. 1993.
16- Morris RJ and Woodcock JP. Intermittent pneumatic compression for bariatric patients – the DVT60 compression garment. Arjo Clinical Report 2003.
17- Falck-Ytter Y, Francis CW, Johanson NA et al. Antithrombotic Therapy and Prevention of Thrombosis, 9th edition: ACCP Evidence Based Clinical Practice Guidelines: Prevention of VTE in Orthopedic Surgery Patients. Chest. 2012; 141: S2.
18- Delince P. RCT of intermittent pneumatic compression (IPC) versus low molecular weight heparin (LMWH) plus anti-embolic stockings (AES) in the prevention of venous thromboembolism during elective hip and knee surgery. Am. Ass. Orth. Surg. Conference 2011.
19- Ginzburg E, Cohn S, Lopez J et al. Randomised clinical trial of intermittent pneumatic compression and low molecular weight heparin in trauma. British Journal of Surgery. 2003; 90: 1338- 1344.
20- Montgomery JS and Wolf JS (2005). Venous Thrombosis Prophylaxis for Urological Laparoscopy: Fractionated Heparin versus Sequential Compression Devices. The Journal of Urology. 2005; 173: 1623-1626.
21- Brooks PJ, Keramati M, Wickline A . Thromboembolism in patients undergoing total knee arthroplasty with epidural analgesia. Journal of Arthroplasty. 2007; 22(5): 641-643.
22- Kurtoglu M, Guloglu R, Ertekin C et al. Intermittent pneumatic compression in the prevention of venous thromboembolism in high-risk trauma and surgical ICU patients. Turkish Journal of Trauma & Emergency Surgery. 2005; 11(1): 38-42.
23- Kurtoglu M, Yanar H et al. Venous thromboembolism prophylaxis after head and
spinal trauma: Intermittent pneumatic compression devices versus low molecular weight heparin. World Journal of Surgery. 2004; 28(8): 807-811.
24- Kamran SI, Downey D and Ruff RL. Pneumatic sequential compression reduces the risk of deep vein thrombosis in stroke patients. Neurology. 1998; 50(6): 1683- 1688.
25- Capper C. External pneumatic compression therapy for DVT prophylaxis. British Journal of Nursing. 1998; 7(14): 851-854.
26- Eskander M, Limb D, Stone M et al. Sequential mechanical and pharmacological thrombo prophylaxis in the surgery of hip fractures. International Orthopaedics. 1997; 21: 259-261.
27- KakkosSK, Caprini JA, Geroulakos G, et al.Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database of Systematic Reviews. Wiley & Sonds. 2016; 9. www.cochranelibrary.com
28- Eppsteiner RW, Shin JJ, Johnson J, van Dam RM. Mechanical compression versus heparin therapy in postoperative and post trauma patients: a systematic review and meta-analysis. World Journal of Surgery. 2010; 34(1): 10-19.
29- Morris RJ, Woodcock JP. Intermittent pneumatic compression or graduated compression stockings for deep vein thrombosis prophylaxis? A systematic review of direct clinical comparisons. Annals of Surgery. 2010; 251(3): 393-6.
30- Elpern E, Killeen K, Patel G, Senecal PA. Original Research: The Application of Intermittent Pneumatic Compression Devices for Thromboprophylaxis. AJN The American Journal of Nursing. 2013 Apr 1;113(4):30-6
31- ECRI Institute. March 2017 https://www.ecri.org/Pages/default.aspx
32- Van Blerk D. Evaluating an Intermittent Compression System for Thromboembolism Prophylaxis. Professional Nurse. 2004; 20(4): 48-49.
33- Pagella P, Cipolle M, Sacco E et al. A randomised trial to evaluate compliance in terms of patient comfort and satisfaction of two pneumatic compression devices.
Orthopaedic Nursing. 2007; 26(3): 169-174.
34- Proctor MC, Greenfield LJ, Wakefield TW et al. A clinical comparison of pneumatic compression devices: the basis for selection. Journal of Vascular Surgery. 2001; 34(3): 459-464.